Population growth, climate change, and increasing standards of living are stressing our supplies of critical resources such as water and metals. Water scarcity is a seminal global challenge, with two-thirds of the world’s population already facing severe water scarcity for at least one month per year. Metals play essential roles in society, but can have environmental and human toxicity concerns. Additionally, many metals (e.g., electronics waste) are negligibly recycled. There is a profound need for innovations that allow for more environmentally responsible and cost-effective management of water and metals.
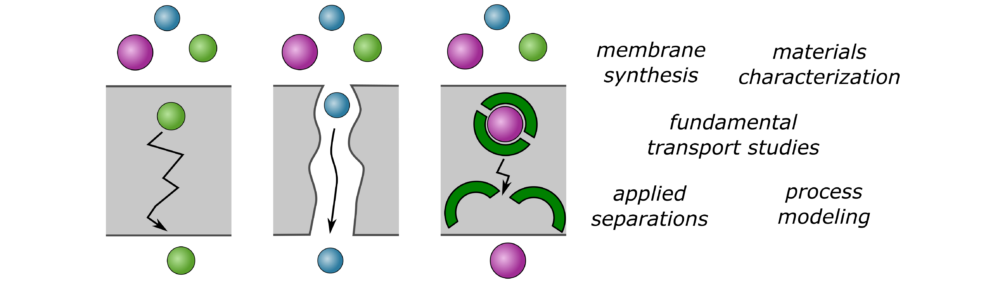
Tremendous opportunity exists in the design of new and improved separation technologies. For example, separations currently account for 10-15% of the global energy consumption. The Advanced Membranes (AM) Lab focuses on membrane separations, with particular emphasis on the design and synthesis of membrane materials that could enable improved and unique selectivities. Such materials could allow for improved operation of important processes—such as reverse osmosis desalination, the premier desalination technique—or could enable entirely new ones.
We have the following long-term goals:
- Revolutionize membrane science R&D through the use of self-driving labs
- Develop ultra-stable membranes to enable energy-efficient dewatering of essentially all aqueous solutions (replace energy-intensive evaporation)
- Enable selective ion separations through the development of ultra-selective membranes (replace solvent extraction)
- Develop sustainable (membrane-driven) processes for purifying bio-derived chemicals
Each of these long-term goals is described below. Our work is highly interdisciplinary and relatively heavy on materials synthesis. Students in our lab have had backgrounds in chemical engineering, chemistry, environmental engineering, hydrometallurgical engineering, and mechanical engineering.
Self-Driving Labs for Membrane Science
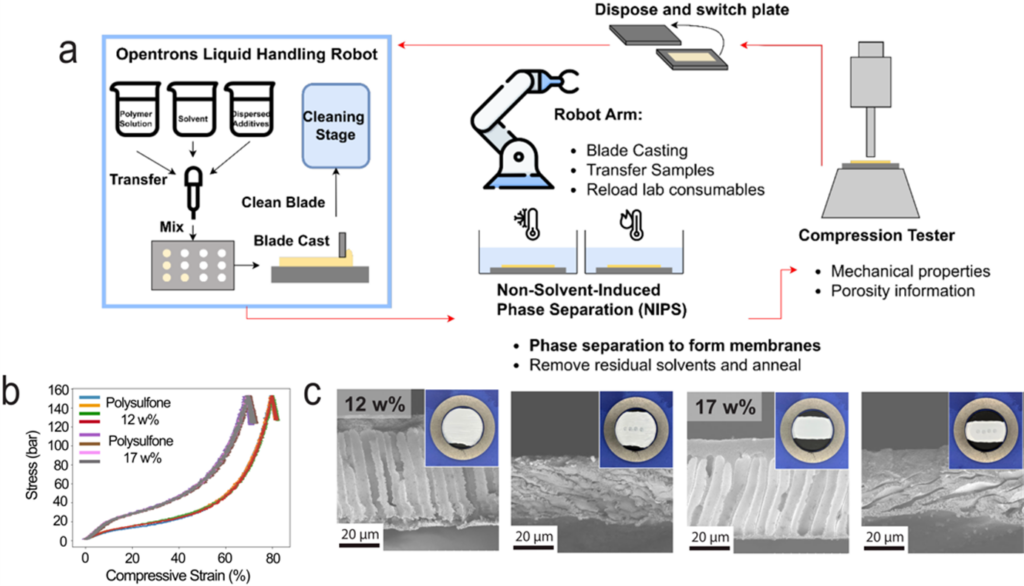
The design of new membranes is highly complex, involving many intersecting parameters. Traditional membrane R&D is manual and slow. Our lab is moving more-and-more towards AI-driven research and the design and use of self-driving labs (SDLs), in which membranes would be synthesized automatically and characterized automatically, after which the “brain” of the SDL chooses the next membrane composition to synthesize.
Our main collaborator in this work is Prof. Jason Hattrick-Simpers in Materials Science and Engineering, who is an expert in AI, robotics, and SDLs. Our work is part of the Acceleration Consortium, which is centered here at UofT. We also collaborate extensively with the Polymers SDL in the Acceleration Consortium, for which Jay is a faculty advisor.
We are currently building three self-driving labs. 1) An SDL for porous membrane fabrication through non-solvent induced phase separation (Hongchen Wang). The system is largely built (YouTube link), and in the next few years, we will use it to optimize the design of porous membranes. 2) An SDL for polymeric “solid carrier-based membranes” (SCBMs) for highly selective ion separations (e.g., Co/Ni). 3) An SDL for interfacial polymerization to synthesize polymeric nanofilms as membrane selective layers.
Related Publications:
- Wang, H.; Zeinali Danalou, S.; Zhu, J.; Sulimro, K.; Lim, C.; Basak, S.; Tai, A.; Siriwardana, U.; Hattrick-Simpers, J.; Werber, J.R. Developing and Validating a High-Throughput Robotic System for the Accelerated Development of Porous Membranes. Journal of Membrane Science 2025, In Press. Pre-print: https://arxiv.org/abs/2508.10973
Ultra-Stable Desalination Membranes to Replace Evaporation
Reverse osmosis (RO) is the best desalination technology when it can be applied. It is modular, energy-efficient, and extremely cost-effective at just $0.40/m3 of fresh water for seawater desalination. For comparison, Toronto’s potable water cost is $4/m3. For many important industrial applications though, RO cannot be applied because the membranes are not stable, typically because of high salinities (which require ultra-high pressures) and extreme pH (which degrade conventional polyamide selective layers). For water recovery, these applications therefore need to use evaporators, which consume enormous amounts of energy owing to the high latent energy of evaporation of water.
Key examples we are targeting include:
– Black liquor production in pulp and paper. Evaporation of this solution uses 1% of total energy in the USA. Black liquor is typically hot (80 °C) and alkaline (pH >12).
– Concentrating hydrometallurgical streams in the metals & mining sector. Hydrometallurgical process waters are typically very low pH (pH ~ 0). Energy-efficient dewatering of streams like sulfuric acid would be a game-changer for enabling circular hydrometallurgy.
– Brine dewatering for inland desalination. Brine disposal is often the most expensive step for inland desalination.
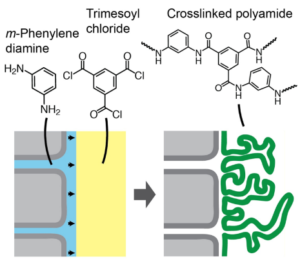
There are two membrane needs to enable osmotic desalination of these solutions. First, extremely compression-resistant membranes are needed to extend the range of RO or to enable new RO variants like “osmotically assisted RO” (OARO). OARO would be the most energy-efficient way to treat brines, if membranes can be made. Second, novel ultra-thin selective layers are needed that employ extremely hydrolytically stable chemistries, but still have water permeability and water/salt selectivities comparable to current membranes. Membranes that combine all of the above are our goal, which could revolutionize water treatment.
Related Publications:
- Sarker, N. R.; Stipanic, D. F.; Petrocelli, I.; Ruiz-Torres, C. A.; Riet, J. A.; Price-Roberts, A.; Werber, J. R. Leveraging Liquid–Liquid Interfaces in a 3D-Printable Reactor to Form Sub-Micron Freestanding Membrane Selective Layers. ACS Appl. Mater. Interfaces 2025, 17 (39), 55360–55371. https://doi.org/10.1021/acsami.5c13451
- Chamani, H.; Rabbani, A.; Russell, K. P.; Zydney, A. L.; Gomez, E. D.; Hattrick-Simpers, J.; Werber, J. R. Data-Science-Based Reconstruction of 3-D Membrane Pore Structure Using a Single 2-D Micrograph. Journal of Membrane Science 2023, 678, 121673. DOI: 10.1016/j.memsci.2023.121673
Membranes and Processes for Selective Ion Separations and Chemical Regeneration
Techniques such as nanofiltration and electrodialysis can be used to separate ions from each other. However, ion/ion selectivity of membranes is often inadequate. For cation/cation selectivity, monovalent/divalent selectivity can be quite high in nanofiltration, and is currently quite poor in electrodialysis. Selectivity between ions of the same valence is typically negligible. For such separations (e.g., Li+/Na+, Co2+/Ni2+), solvent extraction is used industrially, where lipophilic extractants form selective complexes with target ions, bringing them into an organic phase. Sulfuric acid (1-5 M) is typically used to strip (back-extract) the complexed ions. Solvent extraction is commonly used in hydrometallurgy, but has numerous processing and sustainability challenges.

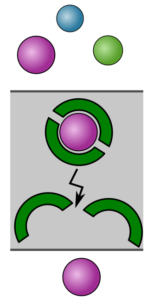
We are interested in scalable paths towards energy-efficient chemical regeneration (e.g., acid and base regeneration from salts through bipolar membrane electrodialysis) and extremely selective ion separations using membranes. These technologies could enable new paradigms for critical minerals production and wastewater treatment.
On-going projects in the group include:
– High-performance monovalent-selective ion exchange membranes
– Highly selective ion exchange membranes for brine electrodialysis
– High-performance bipolar membranes. Current BPMs are expensive and/or inefficient.
– Development of what we are calling “solid carrier-based membranes” (SCBMs), which use diffusing extractants within a polymer matrix. Selectivities with SCBMs are commonly >300 (e.g., for Co/Ni), compared to negligible selectivity (~1) using commercial membranes.
– Membrane development for ion-selective electrodialysis (e.g., Li/Na, Co/Ni)
– Use of BMED for ocean alkalinity enhancement, a promising atmospheric CO2 removal technique
Related Publications:
- Abu-Obaid, S.; Riet, J. A.; Sarker, N. R.; Stipanic, D. F.; Zhu, J.; Gutierrez, R. L.; Ruiz-Torres, C. A.; De Lannoy, C.-F.; Werber, J. R. Thiol–Ene Photopolymerization for Highly Selective Anion Exchange Membranes with Sulfonium and Quaternary Ammonium Fixed Charges. Chem. Mater. 2025, acs.chemmater.5c01307. DOI: https://doi.org/10.1021/acs.chemmater.5c01307
- Ferella, F.; Suichies, A.; Abdelkader, B. A.; Dabhi, N. K.; Werber, J.; De Lannoy, C.-F. Ocean Alkalinity Enhancement Using Bipolar Membrane Electrodialysis: Technical Analysis and Cost Breakdown of a Full-Scale Plant. Ind. Eng. Chem. Res. 2025, 64 (13), 7085–7099. DOI: https://doi.org/10.1021/acs.iecr.4c04364
- Ruiz-Torres, C. A.; Zhu, J.; Riet, J. A.; Sarker, N. R.; Abu-Obaid, S.; Yuan, K. D.; De Lannoy, C.-F.; Werber, J. R. Ultra-Thin Cation Exchange Membranes: Sulfonated Polyamide Thin-Film Composite Membranes with High Charge Density. Chem. Mater. 2024, 36 (22), 11217–11226. DOI: 10.1021/acs.chemmater.4c02270
Develop sustainable (membrane-driven) processes for purifying bio-derived chemicals
Chemicals are mostly produced using fossil fuels; alternatives are clearly needed. The Microbiome Engineering Laboratory, led by Prof. Chris Lawson, are using anaerobic digestion to synthesize valuable chemicals from organic wastes. Their main target are medium-chain fatty acids (MCFAs; carboxylic acids with 6-12 carbons), which have value as animal feed additives, chemical precursors, and ingredients in cosmetic formulations. Our team is leading the development of sustainable purification processes for MCFAs. This is an academic pursuit, but also a potentially commercial one, as we are scaling up the processes to pilot-scale and beyond. Thus far, our focus has been on using silicone membranes for robust MCFA extraction, driven by a pH gradient. This technique offers simple, cost-effective, and selective separations of MCFAs from short-chain fatty acids (C2-C5) and other chemicals. Going forward, we will continue to fundamentally investigate this extraction step, explore acid/base regeneration steps, and support scale-up activities.
What is really exciting about this project area is the opportunity to incorporate sustainable separation processes right at the start of a new industry, which is the right time to do so, rather than trying to make the field switch from distillation after columns have already been built. Also, the chemical production is itself highly sustainable.
Related Publications:
- Zhu, J.; Gutierrez, R.L.; Zhang, Y.; Sarker, N.R.; Dyussekenova, D.; Parmar, J.; Kim, B.C.; Abbas, B.; Ren, K.; Lawson, C.E.; Werber, J.R. Selective and Solvent-Free Extraction of Medium-Chain Fatty Acids with Polydimethylsiloxane Membranes. Submitted. Pre-Print link.