Home
The Bender laboratory is a diverse multi-disciplinary research environment. We focus on the development of new and novel materials for application within the organic electronic space. Towards this goal we undertake activities spanning from the fundamentals of applied chemistry to device integration and engineering.
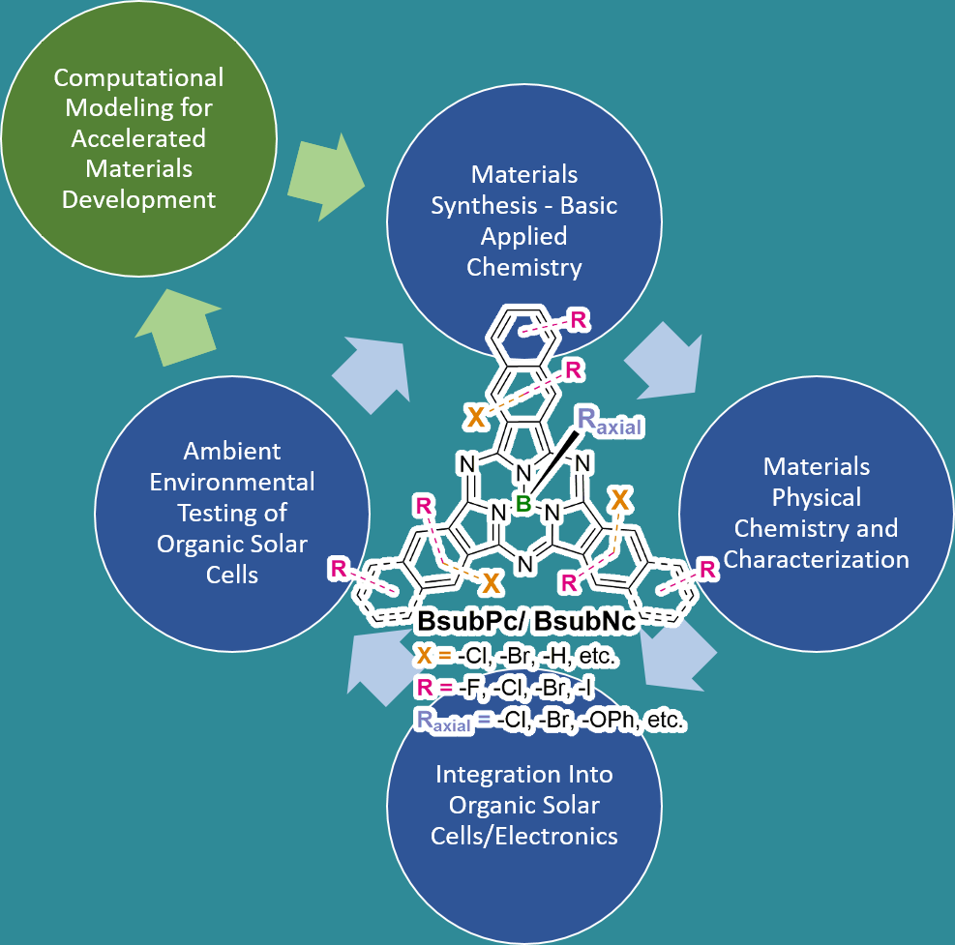
For research towards chemical/materials development and their applications, the Bender laboratory does a loop of computational modeling (to accelerate their development), addresses fundamental and applied chemistry, does physical chemistry characterization to outline appropriate properties, then engineers the materials into devices and then if the general outcome is positive the Bender laboratory also tests the devices in the ambient environment to study the stability and/or aging.
To enable this loop, we have in place the infrastructure to not only conduct complete synthesis, development and purification of organic chemical/materials but to also engineer and consider all of our novel organic electronic materials within the space of organic solar cells (OSCs) and organic light emitting diodes (OLEDs). This means we have the capability to perform fundamental materials design and synthesis and incorporate our new materials into OSCs and OLEDs to assess the functionality and performance.
This allow us to ourselves establish molecular structure property relationships not just for basic physical properties but also for all device metrics achieved via device engineering and environmental testing. The result is a synergistic feedback of device data and metrics to molecular design and synthesis. To achieve the loop, we do not rely on a single HQP to complete this cycle – HQP: highly qualified personals = undergraduate, graduate, post-doctoral fellow – rather HQPs form collaborative teams. For example, an applied chemist will collaborate with a device engineer and/or and materials engineer, each will be coauthors on their respective papers and each will learn from each other. The result is what I refer to as the “applied chemistry – device continuum”. I allow my HQPs to place themselves within this space to reflect their desired educational and research experience. However, there have been several experiences within the lab when an HQP was interested in following the entire loop/cycle – and was/is always acceptable. This research approach applies to every level of HQP from undergraduate students, to graduate students and to post-doctoral fellows. We always recruit/have within the laboratory a set of diverse HQPs including from the space of chemistry, materials science and chemical engineering to consider cross-disciplinary approaches.
Active Lines of Inquiry:
Yes, we are actively developing unique macrocycle compounds for the following set of applications:
- Organic solar cell (also called organic photovoltaics)
- Organic light emitting diodes (OLEDs)
- Battery storage/energy storage
Yes, due to these unique macrocycles, we are also actively:
- Developing fundamental chemistry
- Developing applied chemistry/chemical engineering aspects
- Considering sustainability/developing them as sustainable materials
In the near future, we may also be considering the applications outside of organic electronics:
- As a catalysis (an electro catalysis, a photo catalysis)
Recent Outcomes:
[——]
Retro Outcomes:
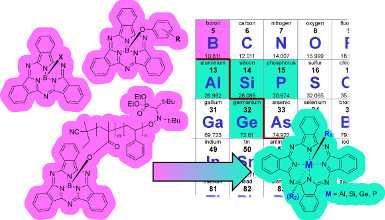
In the past, we had focused on a p-block element array of phthalocyanines to determine the best set applicable to organic electronic devices. Therefore, we have identified boron subphthalocyanines (BsubPcs) and silicon phthalocyanines (SiPcs) as the most applicable phthalocyanines into our desired application. We had also outlined how liquid triaryl amines can also be applied into organic electronics to have a liquid state conduction of electrons.
Retro Notes:
(1) Phthalocyanines as Active Materials in Organic Electronics.
For some time our group has been focused on the design and synthesis of derivatives of boron subphthalocyanine (BsubPc) for application in organic electronics with a specific focus on organic photovoltaics and light emitting diodes. In most cases, organic electronic devices utilize the prototypical BsubPc, chloro boron subphthalocyanine (Cl-BsubPc). We have shown that by using facile chemical transformations we can tailor the nature of the BsubPc to a dye, a sublimate, an engineered crystal or polymer.
Early on in our research program we showed that peripheral functionalization of the BsubPc moiety had detrimental effects on the electrochemical behavior/stability. We therefore focused on chemical derivatization of the boron metal centre while maintaining the hydrogen periphery. The chemical transformations we have found most successfully rely on the oxo-philicity of the boron metal centre. Transformations include phenoxylation and acetylation.
As mentioned above we focus on the application in organic electronic devices including organic light emitting diodes (OLEDs) and organic photovoltaics (OPVs).
Recently we began the exploration of other p-block metal phthalocyanines (MPcs) including Pcs of aluminum, silicon, germanium and phosphorous. In each case the metal was chosen due to its abundance and its ability to participate in our previously established oxo-philic based chemistry. From this group silicon phthalocyanine has emerged as an electron transporting material shown to function in a fullerene free OPV. We have also shown that phenoxylation enhances the performance of silicon phthalocyanine as an electron transporting material.
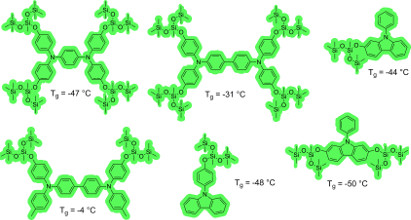
(2) Liquid Triarylamines.
Since initiating this research program six years ago, we found two approaches to the synthesis of liquid triarylamines. Each approach utilizes the unique properties of organosilicon to tailor the physical (viscosity, Tg) and electronic (E1/2,ox) of the triarylamine and each is amenable to systematic variation. The first uses simple silyl ethers and the second uses Piers-Rubinsztajn conditions ((C6F5)3B catalysis) to introduce discrete silicones into the triarylamine structure. Using our methods, a crystalline triarylamine (m.p. = 122 °C) can be transformed into a liquid using either a silyl ether (Tg = -14 °C) or a discrete silicone (Tg = -45 °C). We have shown our liquid triarylamines function in dye sensitized solar cells (DSSCs) with efficiency < 2.4%. We have also shown that they possess higher charge carrier mobilities than their solid crystalline counterparts and that our general methodology can be extended to make liquid polymeric triarylamines. We have published a review article highlighting the intersection of triarylamines and silicon containing materials.