Population growth, climate change, and increasing standards of living are stressing our supplies of critical resources such as water and metals. Water scarcity is a seminal global challenge, with two-thirds of the world’s population already facing severe water scarcity for at least one month per year. Metals play essential roles in society, but can have environmental and human toxicity concerns. Additionally, many metals (e.g., electronics waste) are negligibly recycled. There is a profound need for innovations that allow for more environmentally responsible and cost-effective management of water and metals.
Tremendous opportunity exists in the design of new and improved separation technologies. For example, separations currently account for 10-15% of the global energy consumption. The Advanced Membranes (AM) Lab focuses on membrane separations, with particular emphasis on membrane materials that could enable improved and unique selectivities. Such materials could allow for improved operation of important processes—such as reverse osmosis desalination, the premier desalination technique—or could enable entirely new ones. The AM Lab focuses on water treatment, desalination, ion exchange membranes, and selective ion separations, an area with profound need of technical innovation and with major applications in the mining and metals industries.
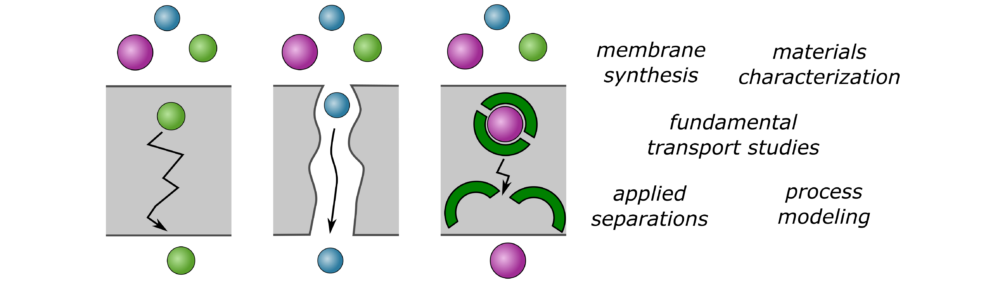
Some initial research areas are described below. All work in the AM Lab combines elements of the following:
- Synthesis and physicochemical characterization of membrane materials
- Fundamental transport studies using lab-scale separations equipment
- Comparison of transport behavior to models to link chemical structure with performance
- Lab-scale separations using model and real-world feed streams
- Process modeling to link membrane properties with process performance
Self-Driving Labs for Membrane Science
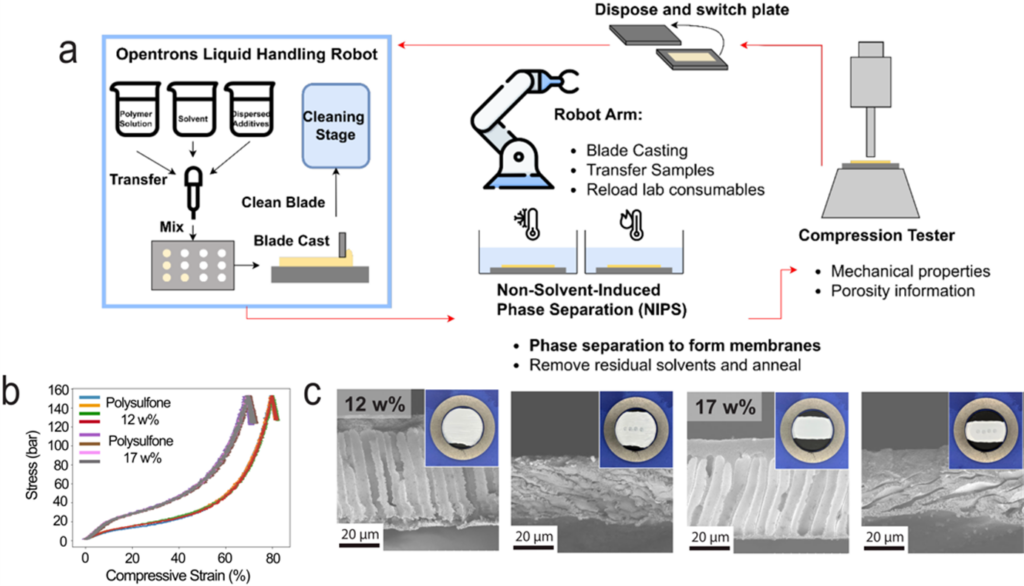
We have a strong collaboration with the group of Prof. Jason Hattrick-Simpers in Materials Science and Engineering on the development of self-driving labs for membrane synthesis and characterization. This is done as a part of the Acceleration Consortium, which is centered here at UofT. We are currently building two self-driving labs: 1) an automated system for porous membrane fabrication (YouTube link), and 2) an automated system for interfacial polymerization of nanofilms.
Polymer Membranes for Reverse-Osmosis Desalination
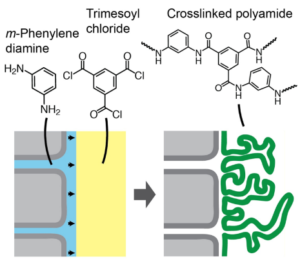
Nearly all industrially used membranes in water and gas separations are polymeric, owing to their ability to be chemically tailored to the target separation, low-cost production, and solution processability. For all membranes, the ability to scalably produce thin, nearly defect-free selective layers is critical to achieving effective separations. Polymers have many advantages in that respect, as simple coating and controlled precipitation methods allow access to selective layers as thin as ~500 nm. Industrially used reverse-osmosis membranes have an even thinner selective layer of just 10–100 nm. These ultra-thin selective layers are formed in situ by polymerizing monomers at an oil-water interface. Work in the AM Lab on polymeric membranes will focus on understanding fundamental relationships between chemical structure and performance, as well as designing new polymeric membranes. Our work will have important implications for industrial membrane usage and design, especially for reverse osmosis.
Related Publications:
- Ritt, C.L.; Werber, J.R.; Wang, M.; Yang, Z.; Zhao, Y.; Kulik, H.J.; Elimelech, M. Ionization behavior of nanoporous polyamide membranes. Proc. Nat. Acad. Sci. U.S.A. 2020, 117, 30191-30200. DOI: 10.1073/pnas.2008421117
- Porter, C.J.; Werber, J.R.; Ritt, C.L; Guan, Y.; Zhong, M.; Elimelech, M. Controlled grafting of polymer brush layers from porous cellulosic membranes. Journal of Membrane Science. 2020, 596, 117719. DOI: 10.1016/j.memsci.2019.117719
- Werber, J.R.; Bull, S.K.; Elimelech, M. Acyl-chloride quenching following interfacial polymerization to modulate permeability and surface charge of desalination membranes. Journal of Membrane Science 2017,535, 357-364. DOI: 10.1016/j.memsci.2017.04.041
- Chen, D.*; Werber, J.R.*; Zhao, X.; Elimelech, M. A facile method to quantify the carboxyl group areal density in the active layer of polyamide thin-film composite membranes. Journal of Membrane Science 2017, 534, 100-108. DOI: 10.1016/j.memsci.2017.04.001
Mesoporous Membranes
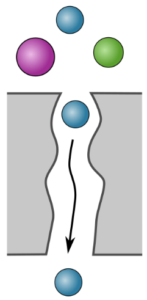
Mesoporous membranes have pores of 2–50 nm in diameter. These membranes are essential for ultrafiltration, a pressure-driven filtration process used to purify water in water-treatment facilities (removing viruses, bacteria, and macromolecules) and water-soluble products in process industries (such as monoclonal antibodies in the biopharmaceutical industry). Ultrafiltration processes must overcome issues with fouling, i.e., the build-up of biological, organic, and inorganic matter on the membrane surface. Tailored surface chemistries are critical in mitigating fouling. Our goal in this area is to develop industrially scalable methods to fabricate high-performance mesoporous membranes with controlled surface chemistry. Such surface chemistries will not only target anti-fouling behavior, but also allow for new membrane applications and separations.
Related Publications:
- Hampu, N.; Werber, J.R.; Chan, W.Y.; Feinberg, E.C.; Hillmyer, M.A. Next-generation ultrafiltration membranes enabled by block polymers. ACS Nano. 2020, 14, 16446-16471. DOI: 10.1021/acsnano.0c07883
- Hampu, N.; Werber, J.R.; Hillmyer, M.A. Co-casting Highly Selective Dual Layer Membranes with Disordered Block Polymer Selective Layers. ACS Appl. Mater. Interfaces. 2020, 12, 45351-45362. DOI: 10.1021/acsami.0c13726
Protein-Based Synthetic Membranes
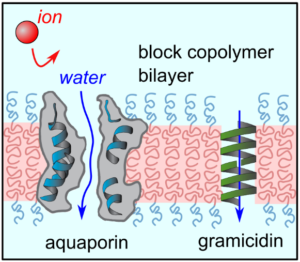
While polymeric membranes offer many advantages, new materials and approaches may be needed for breakthrough performance in desalination and aqueous separations. We are interested in incorporating proteins with unique properties into membrane materials. For example, Jay’s PhD considered the use of aquaporins, membrane proteins that are nature’s water channels. Aquaporins amazingly allow for fast water transport with perfect rejection of solutes. Our early work showed that aquaporin-based membranes could be up to ~10,000,000x more selective than current reverse osmosis membranes. On-going projects in the group involve other fascinating proteins with unique selectivities.
Related Publications:
- Porter, C.J.; Werber, J.R.; Zhong, M.; Wilson, C.J.; Elimelech, M. Pathways and Challenges for Biomimetic Desalination Membranes with Sub-Nanometer Channels. ACS Nano 2020, 14, 10894-10916. DOI: 10.1021/acsnano.0c05753
- Werber, J.R.; Porter, C.J.; Elimelech, M. A path to ultra-selectivity: support layer properties to maximize performance of biomimetic desalination membranes. Environ. Sci. Technol. 2018, 52, 10737-10747. DOI: 10.1021/acs.est.8b03426
- Werber, J.R.; Elimelech, M. Permselectivity limits of biomimetic desalination membranes. Science Advances. 2018, 4, eaar8266. DOI: 10.1126/sciadv.aar8266
- Werber, J.R.; Deshmukh, A.; Elimelech, M. The critical need for increased selectivity, not increased water permeability, for desalination membranes. Environ. Sci. Technol. Lett. 2016,3, 112-120. DOI: 10.1021/acs.estlett.6b00050
- Werber, J.R.; Osuji, C.O.; Elimelech, M. Materials for next-generation desalination and water purification membranes. Nature Reviews Materials 2016, 1, 16018. DOI: 10.1038/natrevmats.2016.18
Facilitated (Carrier) Transport Membranes for Ion-Ion Separations
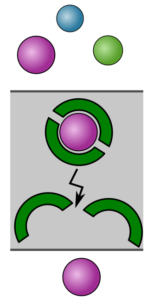
Conventional polymeric and ion exchange membranes separate ions based on size and/or charge. In other words, separating monovalent ions from multivalent ions can be achieved readily using current materials. However, current materials are unable to separate ions of similar size and charge density, such as Cs+ from Na+ or Cu2+ from Ni2+. Facilitated transport membranes offer the potential to achieve these types of separations. These materials take advantage of selective ligands designed for solvent extraction, which form highly selective ligand-ion complexes. The ligands then carry the ion across the membrane, releasing them on the other side. While incredible separations have been achieved in the lab, these materials have not been robust enough for industrial usage. Our work will seek to create robust materials that can achieve unprecedented selectivity for targeted species. Such performance could enable entirely new processes that benefit the mining industry, E-waste recycling, and water treatment.
Related Publications:
- Werber, J.R.; Peterson, C.; Stipanic, D.F.; Hillmyer, M.A. Polymeric Microcapsules as Robust Mimics of Emulsion Liquid Membranes for Selective Ion Separations. Environ. Sci. Technol. 2022. DOI: 10.1021/acs.est.2c07242