Contributions
1. Boronsuphthalocyanines (BsubPcs). My group has made significant contributions to the understanding and application of BsubPcs including the expansion of their process chemistry and the development of BsubPcs as a functional organic materials. For example, we have clarified important discrepancies in the literature regarding BsubPcs, most notably that they are very insoluble (< 10-4 M in most solvents).[55] Conversely we have also showed that variations in the chemical structure of BsubPcs can result in a solubility of > 10-2 M.[35,46] We have developed several novel chemical reactions for the BsubPcs including to yield the phthalimido-BsubPcs, a class can easily be produced which have improved electrochemical stability and are also electrochemically ambipolar.[45] Additionally we also developed a chemical process whereby BsubPcs can be activated to reaction using AlCl3 resulting in the formation of novel derivatives.[36] In parallel we have studied their intrinsic charge carrier mobility and correlated that metric to crystallographic parameters.[43] We have published a review article highlighting both our results and the current state of the field with regards to use BsubPcs as functional materials in organic electronics.[28] The following is what I would consider our most significant contributions in reverse chronological order:
(a) BsubPcs in OSC/OPVs as triplet harvesters.
“Boron subphthalocyanines as Singlet Fission Harvesting Materials within Organic Photovoltaics.” Castrucci, J.S.; Josey, D.; Thibau, E.; Lu, Z-H.; Bender, T.P.*; J. Phys. Chem. Lett., 2015, 6 (15), 3121–3125.
“Acceptor Properties of Boron Subphthalocyanines in Fullerene Free Photovoltaics.” Beaumont, N.; Castrucci, J.S.; Sullivan, P.; Morse, G.E.; Paton, A.S.; Lu, Z.H.; Bender, T.P.*; Jones, T.S.; J. Phys. Chem. C, 2014, 118(27) 14813–14823.
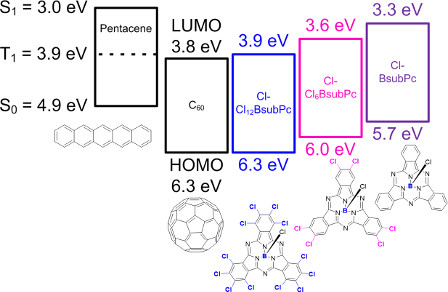
While we have shown that BsubPc are dually functional within an organic solar cell/organic photovoltaic (OSC/OPV)[18] our more recent contributions show that BsubPc synthetic variants can enable the harvesting of triplet excitons from pentacene. The triplet harvesting is significant because this photonic process has the potential to generate more than one electron/electric charge per photon of light absorbed in the OSC and is therefore a mechanism to further push the overall efficiency of OSCs higher than the current benchmarks. In our first paper highlighting this property in 2014, we showed that introduction of six peripheral chlorines to the BsubPc enabled a modest amount of triplet harvesting attributable to the lowering of the HOMO and LUMO energy levels. Within the 2014 paper a hypothesis was offered regarding a molecular design that would further enhance triplet harvesting. In our follow up study published in 2015 we confirmed this hypothesis and showed that a polychlorinated BsubPc can enable triplet harvesting at an efficiency equivalent to or exceeding that of C60, the standard triplet harvesting material. Work is ongoing to further enhance the device performance of this type of BsubPcs as triplet harvesting materials.
(b) Halogen bonding within BsubPcs.
Invited submission for focus issue on Halogen Bonding: “Halogen bonds can direct the solid state arrangement of phenoxy-boron subphthalocyanines.” Virdo, J.D.; Kawar, Y.H.; Bender, T.P.*; CrystEngComm, 2013, 15, 3187-3199.
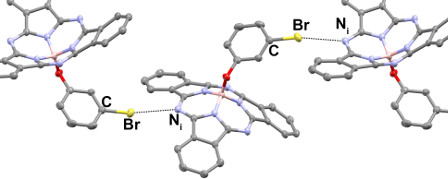
We have established methods to engineer the solid-state arrangement of BsubPcs using a relatively newly discovered and define intramolecular interaction of lower strength than a well-known hydrogen bond known as a halogen bond. In this invited submission we show that the solid-state arrangement for BsubPcs which we have previously shown is dominated by aromatic pi-pi interactions across multiple derivatives, can been circumvented by halogen bonding potential. We have shown this can also be done using hydrogen bonding as well however the use of halogen bonding is of more significance as there is no literature precedence for halogen boning in organic electronics or OSCs. Results to be published shortly, outline how BsubPcs can be used as a platform to test the ability of halogen bonds to be within active materials of OSCs. Our results show that they have limited impact on device performance whereas hydrogen bonds make the OSC non-functional. The BsubPc platform therefore is going to provide the evidence the broader field may cite for the potential of halogen bonds within organic electronic materials.
(c) First BsubPc polymers.
Inside Cover Image: “Boron Subphthalocyanine Polymers: Avoiding the Small Molecule Side Product and Exploring their use in Organic Light Emitting Diodes.” Lessard, B.H.; Sampson, K.L.; Plint, T.; Bender, T.P.*; J. Polym. Sci. A., 2015, 53, 1996–2006.
Highlighted in ‘Materials Views’ and Cover Image: “Boron subphthalocyanine polymers by facile coupling to poly(acrylic acid-ran-styrene) copolymers and the associated problems with autoinitition when employing nitroxide mediated polymerization.” Lessard, B.H.; Bender, T.P.*; Macro. Rapid. Comm., 2013, 34(7), 568-573.
“A Boron Subphthalocyanine Polymer: Poly(4-methyl styrene)-co-poly(phenoxy-boron-subphthalocyanine).” Dang, J.D.; Virdo, J.D.; Lessard, B.H.; Bultz, E.; Paton, A.S.; Bender, T.P.*; Macromolecules, 2012, 45(19), 7791–7798.
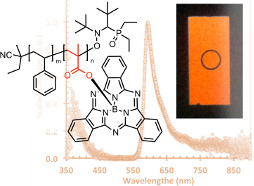
BsubPcs, like normal phthalocyanines, are generally quite insoluble, making their application in solution processed organic electronics not possible. We have used a polymeric backbone to produce soluble poly-BsubPcs. These three papers document our efforts, ultimately resulting in the first application of a polymeric BsubPc in an organic electronic device (OLED). Our approach can be generalized as a methodology for engineering highly insoluble materials into soluble solution processable materials. Additionally, our efforts also identified a fundamental side product of acrylic acid/styrene copolymerization not ever observed or described in the literature.
(d) BsubPcs in OLEDs.
“Pentafluorophenoxy boron subphthalocyanine as a fluorescent dopant emitter in organic light emitting diodes.” Helander, M.H.; Morse, G.E.; Qiu, J.; Castrucci, J.; Bender, T.P.*; Lu, Z.H.; ACS Appl. Mater. & Inter., 2010, 11(2), 3147–3152.
“Fluorinated Phenoxy Boron Subphthalocyanines in Organic Light-Emitting Diodes” Morse, G.E.; Helander, M.H.; Maka, J.; Lu, Z.H.; Bender, T.P.*; ACS Appl. Mater. & Inter., 2010, 2(7), 1934–1944.
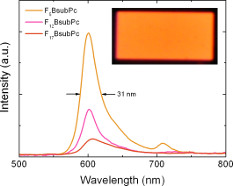
Early on in our research program We showed that the high fluorescence quantum yields of BsubPcs translate into good electroluminescence in OLEDs with a pure orange colour and small peak width at half height of 40 nm. This work has not been stagnant for 5 years, rather, we have been working to leverage the pure orange electroluminescence of the BsubPc and engineering OLEDs with emission spectra equivalent to that of an incandescent light bulb. Publication in preparation.
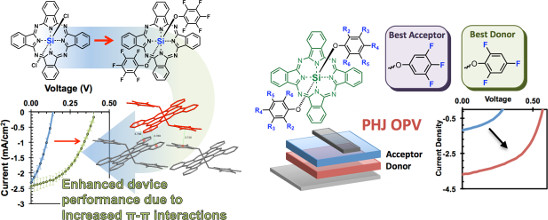
2. Silicon phthalocyanines (SiPcs) in OSCs/OPVs.
“The Position and Frequency of Fluorine Atoms Changes the Electron Donor/Acceptor Properties of Fluorophenoxy Silicon Phthalocyanines within Organic Photovoltaic Devices” Lessard, B.H.; Grant. T.; White, R.; Thibau, E.; Lu, Z-H.; Bender, T.P.*; J. Mater. Chem. C., 2015, accepted and under revision.
“Assessing the Potential Roles of Silicon and Germanium Phthalocyanines in Planar Heterojunction Organic Photovoltaic Devices and How Pentafluoro Phenoxylation Can Enhance π–π Interactions and Device Performance” Lessard, B.; Plint, T.; Castrucci, J.; White, R.; Josey, D.; Lu, Z.H.; Bender, T.P.*; ACS Appl. Mater. Inter., 2015, 7(9), 5076-5088.
We have been working to explore other p-block phthalocyanines for applications in organic electronics. After exploring all group 13 and 14 phthalocyanines, silicon phthalocyanine (SiPc) emerged as a material that can be engineered to be either a hole- or electron-transporting material as well as a strong light absorber. We have shown that our simple phenoxylation chemistry, developed originally for BsubPcs, enables significant performance enhancements in OSC device performance. The cited papers are the first two publications regarding the application of SiPcs in planar heterojunction OSCs.