Research Direction
Our research provides molecular solutions and develops catalytic conversion technology for addressing the challenges in the fields of environment, health, medicine, and energy. We adopt a multidisciplinary strategy (Figure 1) that integrates materials synthesis, spectroscopy, electronic structure theory, and kinetic and isotopic assessments to deepen the fundamental understanding of molecular scale physical and chemical events during chemical transformations.As part of our rigorous efforts in establishing structure and kinetic property correlations, we synthesize novel catalytic materials with well-defined atomic connectivity and compositions and then probe their structures and kinetic consequences. These fundamental studies on reaction kinetics and catalytic surfaces are carried out in parallel with microchemical reactor development and technology demonstration projects. The details of our facility are provided
here. Specific research themes are summarized in the followings.
Renewable Energy: Designing Chemical Conversion Processes for a Bio-Refinery
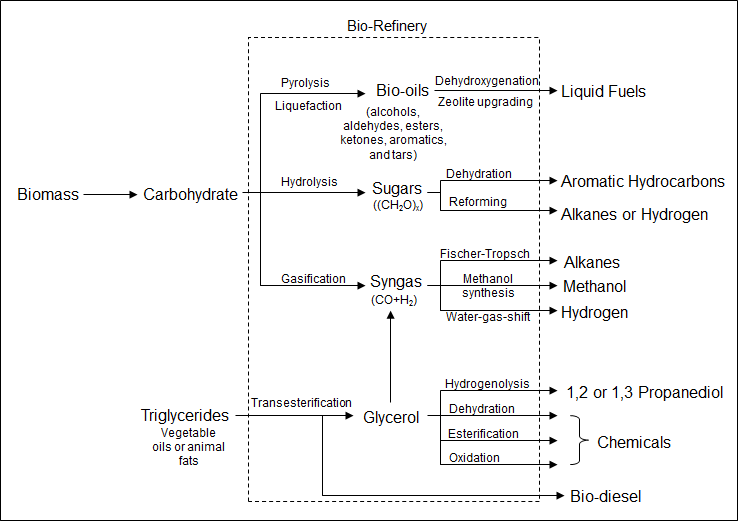
Bio-refineries (Figure 2) are a set of chemical processes that take carbon, oxygen, and hydrogen building blocks from plant biomass and reconstruct these molecular fragments to fuels and valuable chemicals. Such refineries are promising technologies for the next-generation energy cycle using renewable feedstocks to replace fossil fuels. Because of the structural complexity of biomass crude oils, conventional refining technology that has evolved around fossil fuel processing is no longer suitable for processing these compounds. New methods are sought to remove the oxygen heteroatoms from the biomass derived molecules and convert them to H
2, alkanes, and liquid fuels. Also of interest are novel catalytic processes for downstream chemical syntheses using the biomass derived building blocks. We design catalytic materials for selective cleavage of chemical bonds and efficient shuffling of chemical functional groups between molecules. The objective is to develop practical catalytic methods for production of H
2, fuels for transportation, and other chemicals.
Developing “Chemical-Plant-on-a-Chip” Microchemical Reactors
“Chemical-plant-on-a-chip” is a concept of miniaturizing conventional unit operations such as reactors and heat exchangers from macroscopic to microscopic length scales and integrating these units into compact chemical plants as small as the size of a semiconductor chip. Within this context, we design and fabricate microscale reactors and then assess their performances for catalytic processing of oxygenates and fuels under industrially relevant conditions. Such reactors contain micro-structured catalysts with hybrid features from the nano to mesoscopic length scales, which are made by precision engineering and microfabrication methods. The design speeds transport processes by reducing the characteristic lengths for heat and mass transport by several orders of magnitude relative to conventional reactors. We explore a variety of synthesis methods to build molecular architectures such as carbon nanotubes and nano-sized metal particles on the catalysts and then evaluate their performances for catalytic reactions. Figure 3 shows an example of the microstructured catalysts that is made with well-aligned carbon nanofibers whose surfaces are grafted with nano-sized metallic clusters. Such structured catalysts offer large interfacial areas between the fluid and solid catalysts and provide efficient heat removal while maintaining a low pressure drop.
Capturing the Dynamics of Catalytic Sites and their Kinetic Consequences during Chemical Turnovers
Catalytic surfaces undergo dynamic reconstruction during chemical reactions because their surface free energies vary with the composition of the contacting fluid phase. In particular, nanosized metal and alloy clusters undergo dynamic shape changes in response to reaction conditions such as the fluid phase composition and temperature. Understanding the dynamics of catalytic surfaces is critical for rational design of molecular features that enable the reaction. We provide this knowledge of surface dynamics at the molecular scale and their consequences during catalysis using a combined kinetic and isotopic methods and
in-situ infrared and X-ray absorption spectroscopies. These molecular descriptions of surface dynamics are important for developing stable and functional materials for efficient fuel and chemical processing.
![(a) A reactor module consists of millimeter sized reaction channels [1].](../files/2011/09/fpa.png)
(a) A reactor module consists of millimeter sized reaction channels [1].
|
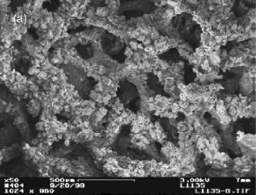
(b) Each of the reaction channels in (a) are loaded with an FeCrAlY metal foam based engineered catalyst; the foam has three dimensional interconnected pores (80 ppi pore density), where the surfaces of the foam are coated with aligned carbon nanotubes. This enables efficient heat and mass transports while maintaining a low pressure drop (SEM image taken at ×50).
|
![(c) High resolution SEM image (×20 000) on the carbon nanotubes that are grown on the FeCrAlY substrate. A layer of microporous solid encapsulated with active sites is grafted onto the surfaces of the carbon nanotubes [2].](../files/2011/09/fpc.png)
(c) High resolution SEM image (×20 000) on the carbon nanotubes that are grown on the FeCrAlY substrate. A layer of microporous solid encapsulated with active sites is grafted onto the surfaces of the carbon nanotubes [2].
|
|
Figure 3. Microchannel reactor for fuel processing.
|
[1] Patents CA 2560831, U.S. 7084180, U.S. 7208136.
[2] Y-H. Chin, J. L. Hu, C. S. Cao, Y. F. Gao, Y. Wang, Catal. Today 110 (2005) 47.
![(a) A reactor module consists of millimeter sized reaction channels [1].](../files/2011/09/fpa.png)

![(c) High resolution SEM image (×20 000) on the carbon nanotubes that are grown on the FeCrAlY substrate. A layer of microporous solid encapsulated with active sites is grafted onto the surfaces of the carbon nanotubes [2].](../files/2011/09/fpc.png)